
We are happy to announce that the 2025 Antibody Society Research Competition is officially open to student and postdoctoral members of The Antibody Society. This competition is designed to recognize and support the innovative research contributions of early-career scientists in the field of antibody science.
Competition Details:
We invite students and postdocs to submit a summary of their research on any topic related to antibody science. While traditional poster presentations are welcome, we also encourage creative formats, such as videos or other unique presentations.
Relevant Topics Include
- Antibody Engineering/Design
- Antibody Therapeutics
- Fc Effector Functions
- Bispecific Antibodies
- Antibody-Drug Conjugates
- Adaptive Immune Receptor Repertoires
Prizes for Winners:
Two winners (1 student and 1 postdoc) will be selected based on originality, creativity, scientific merit, and clarity. Each winner will receive:
- A $400 cash prize
- Free registration for either:
- Schrödinger’s online course,
- Introduction to Computational Antibody Engineering OR
- Virtual Antibody Engineering & Therapeutics
Submission Guidelines:
- Open only to student and postdoc members of The Antibody Society
- Submissions must be your own work, but collaborative research is allowed with permission from co-authors
- Submissions may include unpublished work or content from papers published within the past six months (with proper copyright permissions)
- By entering the competition, winners agree to have their work published on The Antibody Society’s website
Important Dates:
- Start Date: February 1, 2025
- Submission Deadline: April 15, 2025
Please note that only the first 30 submissions will be considered, so we encourage you to submit early. If you’re not yet a member of The Antibody Society, membership is free for students and postdocs, so take this opportunity to join and participate.
To submit your entry or for more information, please email membership@antibodysociety.org.
We look forward to your participation!

 Dr. Jenna Guthmiller is an Assistant Professor in the Department of Immunology and Microbiology at the University of Colorado Anschutz Medical Campus.
Dr. Jenna Guthmiller is an Assistant Professor in the Department of Immunology and Microbiology at the University of Colorado Anschutz Medical Campus.

 The Imaging Calendar Competition ran from April 1st to July 15th 2024 and was open to all members. Participants submitted a high-resolution image with a brief explanation of the research behind it and the scientific method. This year we accepted both wet-lab and in-silico originated images.
The Imaging Calendar Competition ran from April 1st to July 15th 2024 and was open to all members. Participants submitted a high-resolution image with a brief explanation of the research behind it and the scientific method. This year we accepted both wet-lab and in-silico originated images.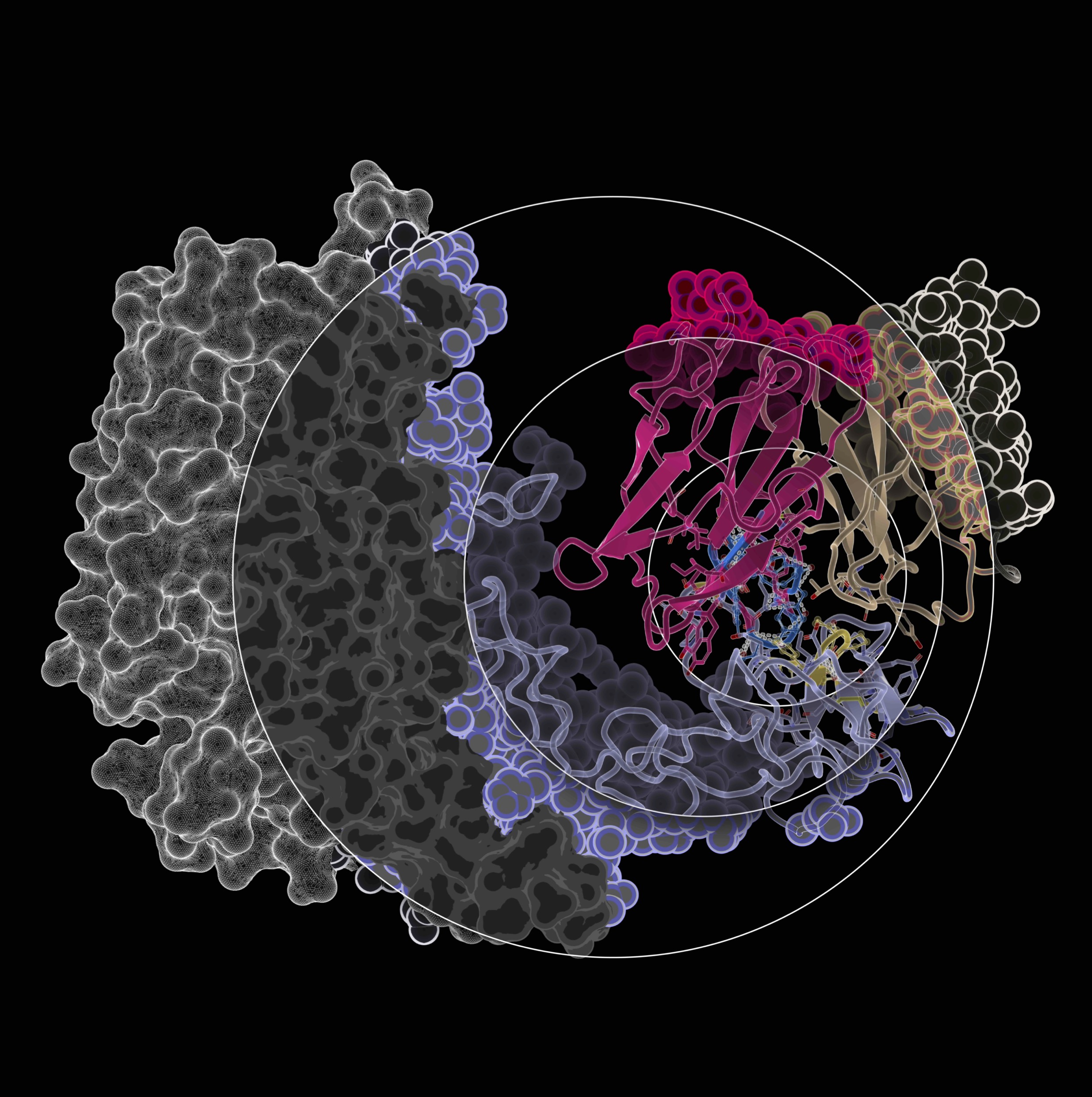

 Our post doc winner is:
Our post doc winner is: