CytoDyn Inc. has submitted the non-clinical portion of a biologics license application (BLA) for leronlimab (700 mg dose) in combination with highly active antiretroviral therapy for treatment of patients with human immunodeficientcy virus (HIV). ‘Rolling’ BLA submission is a benefit of leronlimab’s Fast Track drug designation, which was granted by the US Food and Drug Administration for this indication. CytoDyn is working to complete the clinical, and chemistry, manufacturing and controls sections of the BLA. Leronlimab, a humanized IgG4 monoclonal antibody, blocks CCR5. The chemokine receptor CCR5 is the principal HIV co-receptor, but it has potential as a drug target for other diseases, such as cancer and immune-mediated disorders.
As recently announced by CytoDyn, clinical study data has shown that a weekly dose of 525 mg and 700 mg of leronlimab yielded approximately a 90% response rate for HIV-infected patients who pass the first 10 weeks of monotherapy without virologic failure. Approximately 30% of patients fail within the first 10 weeks of monotherapy on a 525 mg dosage and 17% at a dosage of 700 mg. Patients who pass the first 10 weeks of monotherapy on a 525 mg dose have reached an average total of 32 weeks with sustained viral load suppression.
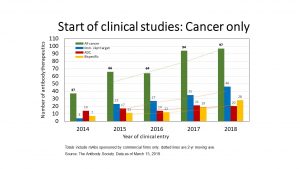
CytoDyn is also developing leronlimab as a treatment for graft-vs.-host disease (GVHD) and triple-negative breast cancer. A Phase 2 study (NCT02737306 ) of the safety and efficacy of leronlimab for prophylaxis of acute GVHD in patients undergoing reduced intensity conditioning allogeneic stem-cell transplantation has an estimated primary completion date of December 2019. A Phase 1b/2 study (NCT03838367) of leronlimab combined with carboplatin in patients with CCR5+ metastatic triple negative breast cancer is not yet recruiting patients.
Like this post but not a member? Please join!
The Antibody Society maintains a comprehensive table of approved mAb therapeutics and those in regulatory review in the EU or US. Please log in to access the table, which is located in the Members Only section and can be downloaded in Excel format. Information about other antibody therapeutics that may enter regulatory review in 2019 can be found in ‘Antibodies to watch in 2019’.