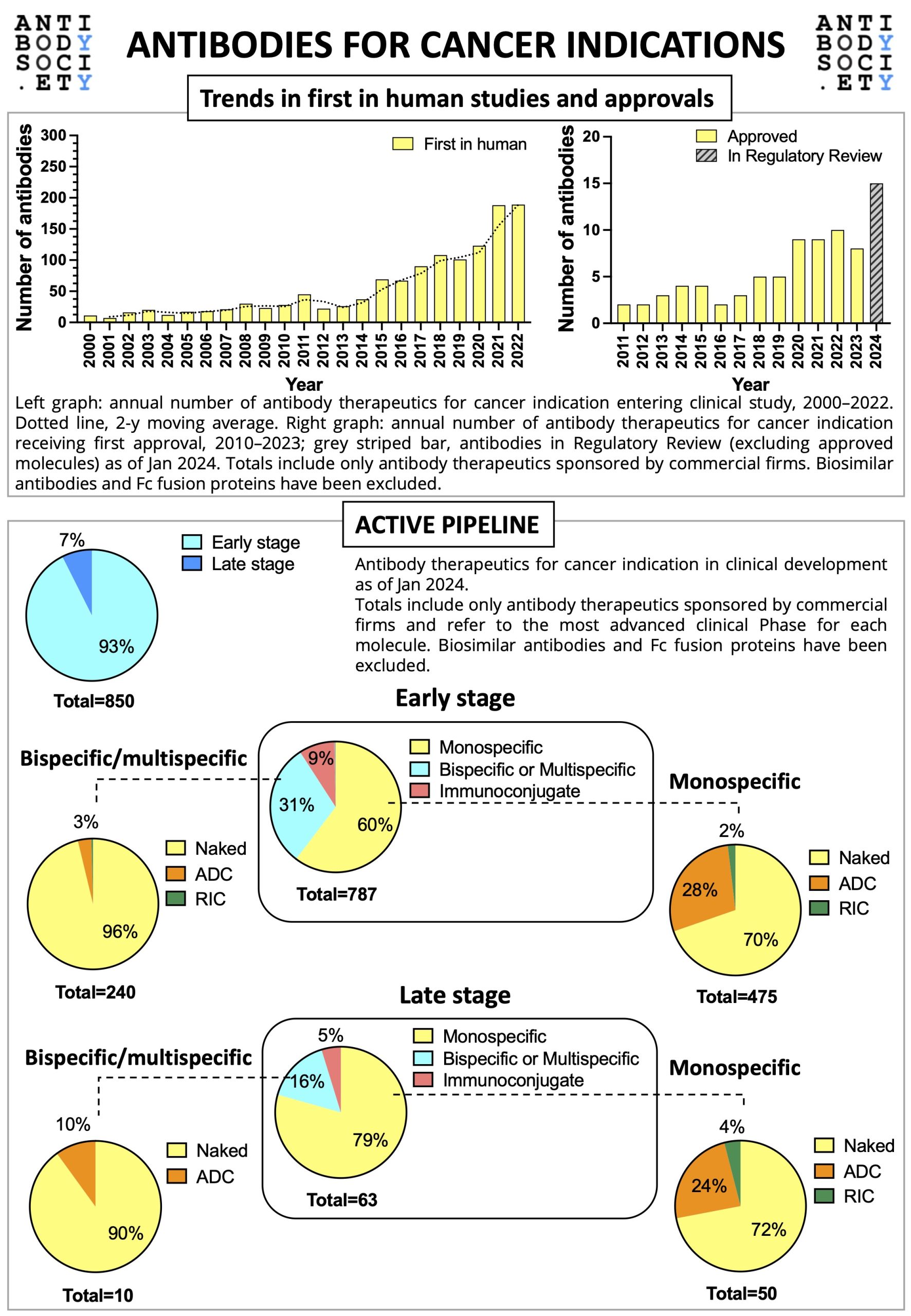
The Antibody Society’s latest analysis of the current early-stage clinical pipeline of antibody therapeutics shows that the biopharmaceutical industry is engaging in innovative research and development. For both cancer and non-cancer indications, ~60% of the antibody therapeutics in early-stage clinical development target novel antigens, and a growing portion of those against well-validated targets have been designed in novel formats (e.g., bispecifics, bispecific biparatopics, antibody-drug conjugates (ADCs), Fc engineered, hybrid isotype, immunoconjugates).
The Antibody Society’s latest analysis of the current early-stage clinical pipeline of antibody therapeutics shows that the biopharmaceutical industry is engaging in innovative research and development. For both cancer and non-cancer indications, ~60% of the antibody therapeutics in early-stage clinical development target novel antigens, and a growing portion of those against well-validated targets have been designed in novel formats (e.g., bispecifics, bispecific biparatopics, antibody-drug conjugates (ADCs), Fc engineered, hybrid isotype, immunoconjugates).
For mAbs’ next Collection, we invite The Antibody Society members, mAbs readers, and the broader scientific community to contribute review articles focused on innovative approaches for antibody therapeutic discovery. The reviews should narrate the state of the art on innovative formats, targets, or platforms for antibody therapeutic discovery, and speculate on new vistas for the field.
mAbs will waive publication charges for up to 8 of the best review articles selected from pre-submission inquiries, which should include the proposed authors, title, abstract, and general outline of the intended review article.
The deadline for pre-submission inquiries is April 1, 2024.
Authors will be notified of the Editors’ decision by April 15, 2024.
The deadline for submission of the completed review articles is December 15, 2024.
We are particularly interested in reviews on the following topics:
- Novel platforms for antibody therapeutic discovery (e.g., novel target/epitope selection, novel approaches for enhancing or reducing Fc effector function, novel methods for enhancing antibody therapeutics half-life).
- Novel targets or formats for cancer immunotherapy (i.e., novel antigens and formats for targeting tumors, immune cells, and the tumor microenvironment), design strategies for novel approaches in cancer immunotherapy.
- Novel targets or formats beyond oncology (e.g., autoimmune diseases, cardiovascular/hemostasis, neurology, infectious disease, ophthalmology, rare diseases).
- Novel immune-modulating antibodies (i.e., novel immune checkpoints and immune agonists), relevant design strategies, formats, applications and considerations for safety and tolerability in multiple disease areas.
- Innovative format combinations (e.g., Bispecific/Multispecific ADCs, Bispecific/Multispecific checkpoint inhibitors), design strategies for these molecules and considerations for safety.
- Novel formats for well-validated targets, relevant design strategies and considerations for efficacy, biodistribution and safety.
Although these topics are especially of interest, we welcome well-written reviews in related areas as well.
Please send pre-submission inquiries that include the authors, title, abstract, and general outline of the intended review article to Editor-in-Chief Dr. Janice Reichert (reichert.biotechconsulting@gmail.com) and Guest Editors Drs. Silvia Crescioli (silvia.crescioli@antibodysociety.org) and Kristina Ilieva-Babinsky (k.m.ilieva@gmail.com), and Prof. Jamie Spangler (jamie.spangler@jhu.edu). Please feel free to contact us if you have any questions.
Examples of other mAbs Collections can be found here.