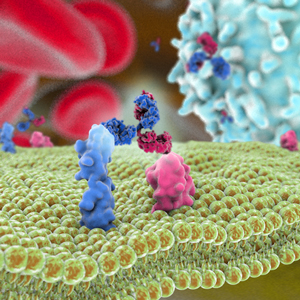
T-cell recruitment for the treatment of cancer has garnered substantial interest over the past thirty years [1,2]. In this type of therapy, activated tumor-specific cytotoxic T-cell lymphocytes are directed to malignant tumor, and subsequently destroy them. Activation of this mechanism relies on T cells and tumor cells being in close proximity to one another. One popular strategy for bringing the cells together involves use of a bispecific antibody [3] where the dual-antigen specificity can enable simultaneous binding of a tumor-specific antigen along with an antigen present on a cytotoxic T-cell. In addition to having the advantage of enhanced functionality compared to a monospecific antibody, garnering dual specificity from single-agent therapy can simplify the development process, e.g., only one molecule needs to be approved. Two bispecific antibodies that take advantage of this principle have been approved: catumaxomab (Removab®) for the treatment of malignant ascites secondary to epithelial cancers and blinatumomab (Blincyto®) as second-line treatment for B cell acute lymphocytic leukemia (ALL).
Catumaxomab is a monoclonal IgG-like antibody [4]. It is termed trifunctional because one of the Fab arms binds epithelial tumor cells via the epithelial cell-adhesion molecule (EpCAM) antigen site, the other Fab arm binds cytotoxic T cells via the CD3 receptor, while the Fc acts as the third site of action, selectively engaging Fcγ receptor I-, IIa- or III on accessary cells. Thusly in this strategy, tumor cell destruction relies not only on T-cell lysis, but also on T-cell activation of accessory cells such as macrophages, dendritic cells and natural killer cells, which engage in destruction of tumor cells by various mechanisms such as perforin-mediated lysis, antibody-mediated phagocytosis and cytokine release. In 2009, catumaxomab became the first bispecific antibody to be approved, for use in Europe [4].
Blinatumomab, in contrast, is a bispecific T cell engager (BiTE) [5]. It is composed of two tandem single-chain variable fragments, each with unique specificity, fused together by a short flexible linker. One arm of this bispecific molecule binds CD3 on T cells while the other arm binds CD19, an antigen found on almost all B-lineage ALL cells and in many places throughout B cell differentiation. Bridging of the two antigens enables T-cell activation and exertion of cytotoxic activity by lysis of target B cells. Two advantages of this bispecific molecule are its small size, which results in fast systemic clearance and ensures close proximity of T cells to target cell, and its flexibility, which is thought to lead to efficient induction of T-cell activation by enabling optimal interaction with target epitopes on the two opposing cell membranes. In some cases, bifunctionality is preferred over trifunctionality because of concern that the Fc receptor interactions could potentially lead to dampening of the immune response. In 2014, blinatumomab became the first bispecific antibody approved for use in the United States. It is currently being evaluated in Phase 2 clinical trials for the treatment of other ALL-related diseases [6].
Nonetheless despite very encouraging preclinical results [7-9] and extensive clinical activities [10-13], additional successful outcomes in the clinic have not been forthcoming. Of the twenty novel bispecific antibodies that entered first-in-humans clinical studies in 2014-2015, approximately half invoke a T-cell recruiting mechanism. Despite this abundance, none of these have advanced beyond Phase 1. Thus far, toxicity and lack of significant anti-tumor response appear to be the primary barriers to advancement of these agents. Improving the selectivity of T-cell activation is being examined as a way to address toxicity issues. To increase the effectiveness of the agents, alternate dose administration strategies are being tested. For example, blinatumomab’s dosing was changed to continuous infusion instead of intravenous injection to ensure continuous activation of T cells against target cells [14]. In addition, use of T-cell recruiting bispecific antibodies as first in-line treatment or as a component of combination therapies are also being evaluated to determine whether significant gains in patient response can be achieved. If such gains can be achieved with these approaches, more T-cell activating bispecific antibodies that will successfully meet patient needs may be available in the future.
1. Staerz UD, Kanagawa O, Bevan M. Hybrid antibodies can target sites for attack by T cells. Nature 1985; 314:628–31.
2. Perez P, Hoffman RW, Shaw S, Bluestone JA, Segal DM. Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature 1985; 316:354–6.
3. Weidle UH, Kontermann RE, Brinkmann U. Tumor-antigen–binding bispecific antibodies for cancer treatment. Seminars in Oncology 2014; 41:653–60.
4. Linke R, Klein A, Seimetz D. Catumaxomab: Clinical development and future directions. mAbs 2014; 2:129–36.
5. Wolf E, Hofmeister R, Kufer P, Schlereth B, Baeuerle PA. BiTEs: bispecific antibody constructs with unique anti-tumor activity. Drug Discovery Today 2005; 10:1237–44.
6. Turner J, Schneider S. Blinatumomab: A new treatment for adults with relapsed acute lymphocytic leukemia. Clin J Oncol Nurs. 2016; 20:165–8.
7. Deo YM, Sundarapandiyan K, Keler T, Wallace PK, Graziano RF. Bispecific molecules directed to the Fc receptor for IgA (FcαRI, CD89) and tumor antigens efficiently promote cell-mediated cytotoxicity of tumor targets in whole blood. J Immunol 1998; 160:1677–86.
8. Löffler A, Kufer P, Lutterbüse R, Zettl F, Daniel PT, Schwenkenbecher JM, Riethmüller G, Dörken B, Bargou RC. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood 2000; 95:2098–103.
9. Heiss MM, Ströhlein MA, Jäger M, Kimmig R, Burges A, Schoberth A, Jauch K-W, Schildberg F-W, Lindhofer H. Immunotherapy of malignant ascites with trifunctional antibodies. Int J Cancer 2005; 117:435–43.
10. Begent RH, Verhaar MJ, Chester KA, Casey JL, Green AJ, Napier MP, Hope-Stone LD, Cushen N, Keep PA, Johnson CJ, et al. Clinical evidence of efficient tumor targeting based on single-chain Fv antibody selected from a combinatorial library. Nat Med 1996; 2:979–84.
11. Burges A, Wimberger P, Kümper C, Gorbounova V. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM× anti-CD3 antibody: a phase I/II study. Clin Cancer Res. 2007; 13:3899-905.
12. De Gast GC, Van Houten AA, Haagen IA, Klein S, De Weger RA, Van Dijk A, Phillips J, Clark M, Bast BJ. Clinical experience with CD3 X CD19 bispecific antibodies in patients with B cell malignancies. J Hematother. 1995;4:433-7.
13. Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, Dudnichenko AS, Aleknaviciene B, Razbadauskas A, Gore M, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int J Cancer 2010; 127:2209–21.
14. Klinger M, Brandl C, Zugmaier G, Hijazi Y, Bargou RC, Topp MS, Gökbuget N, Neumann S, Goebeler M, Viardot A, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell–engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood 2012; 119:6226–33.